This last introduction chapter will introduce you to Gel Electrophoresis, a method to separate samples of DNA fragments by their size.
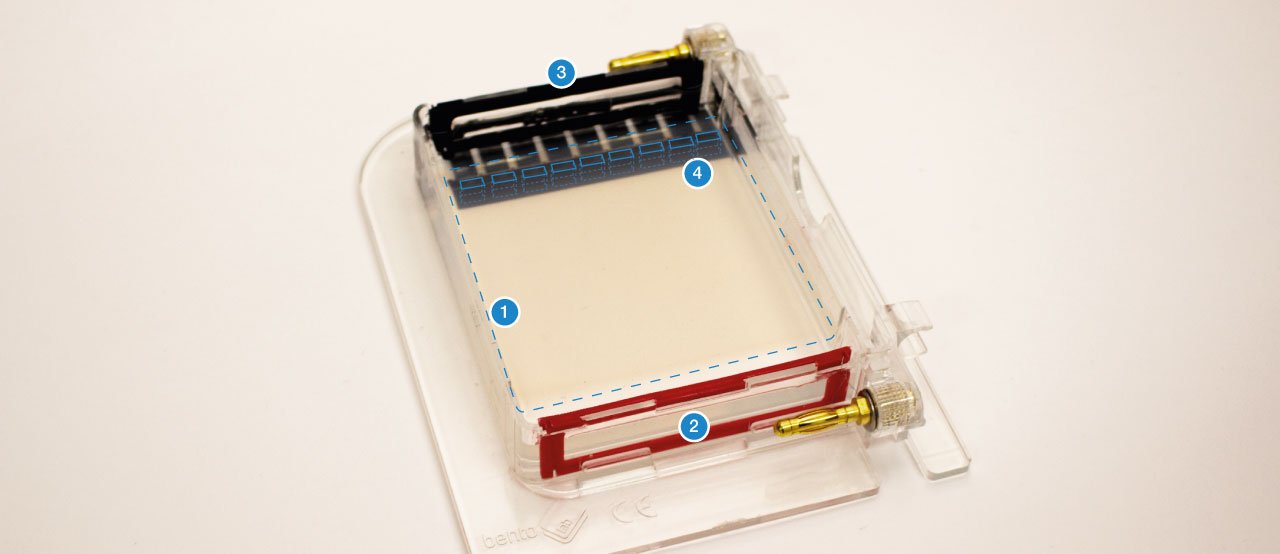
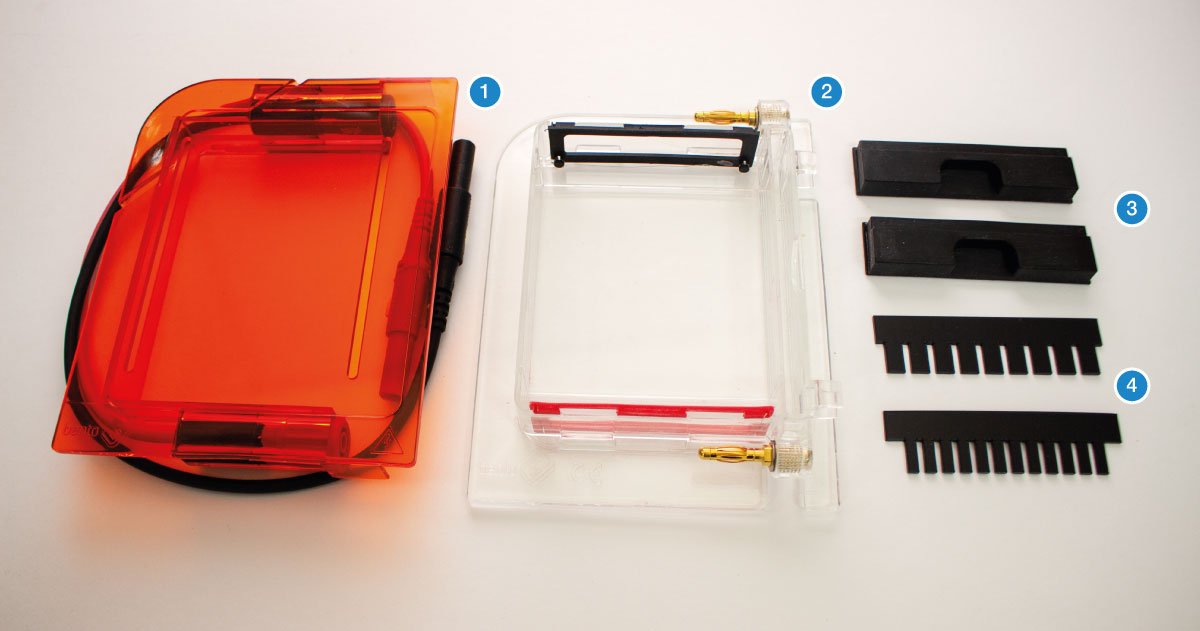
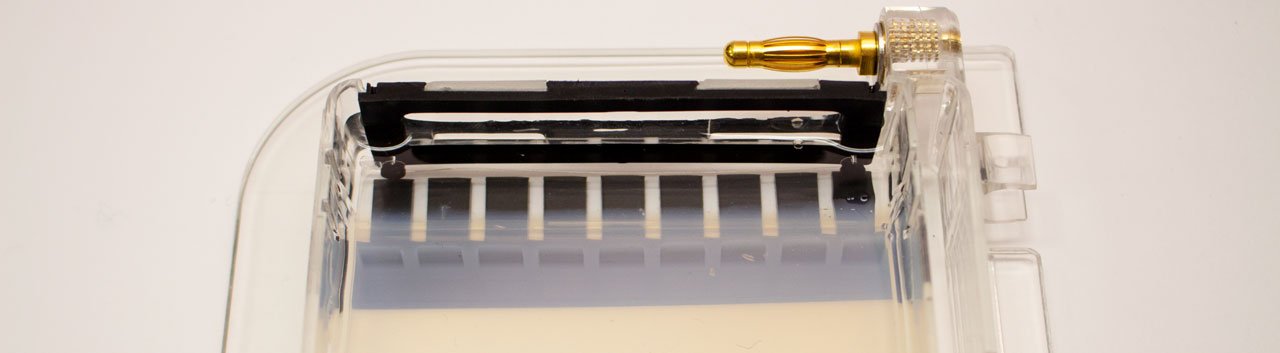
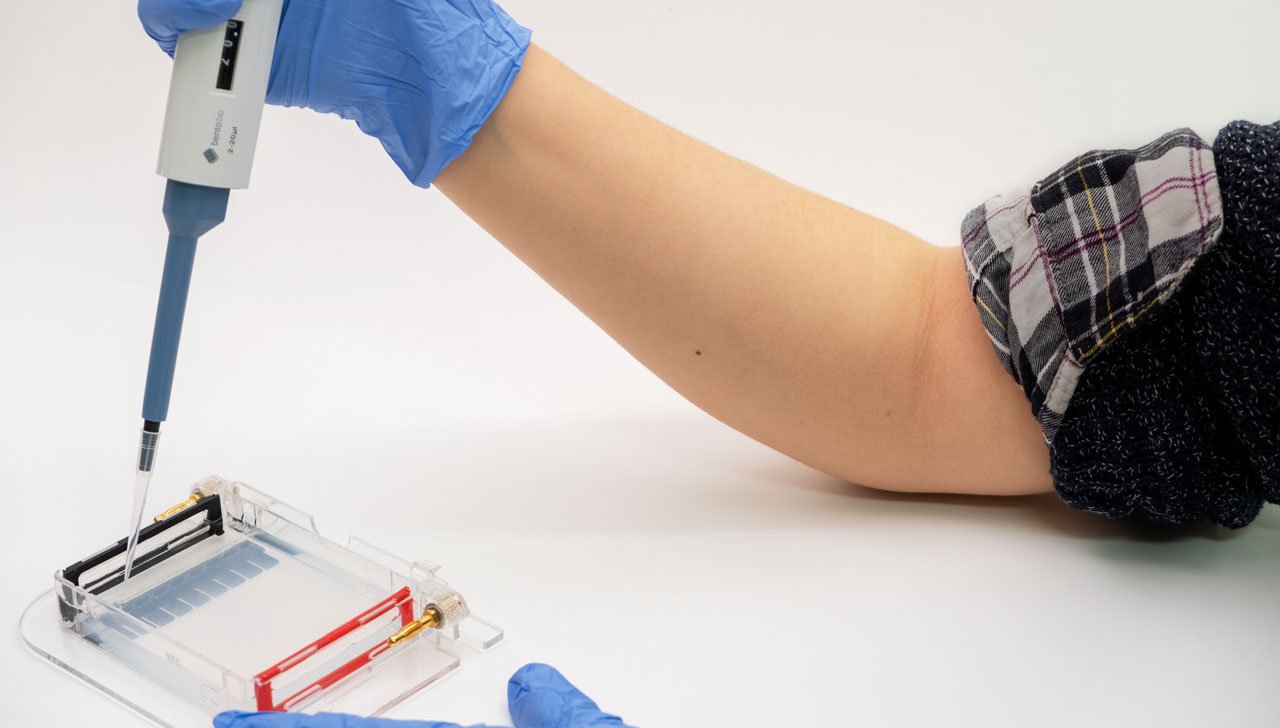
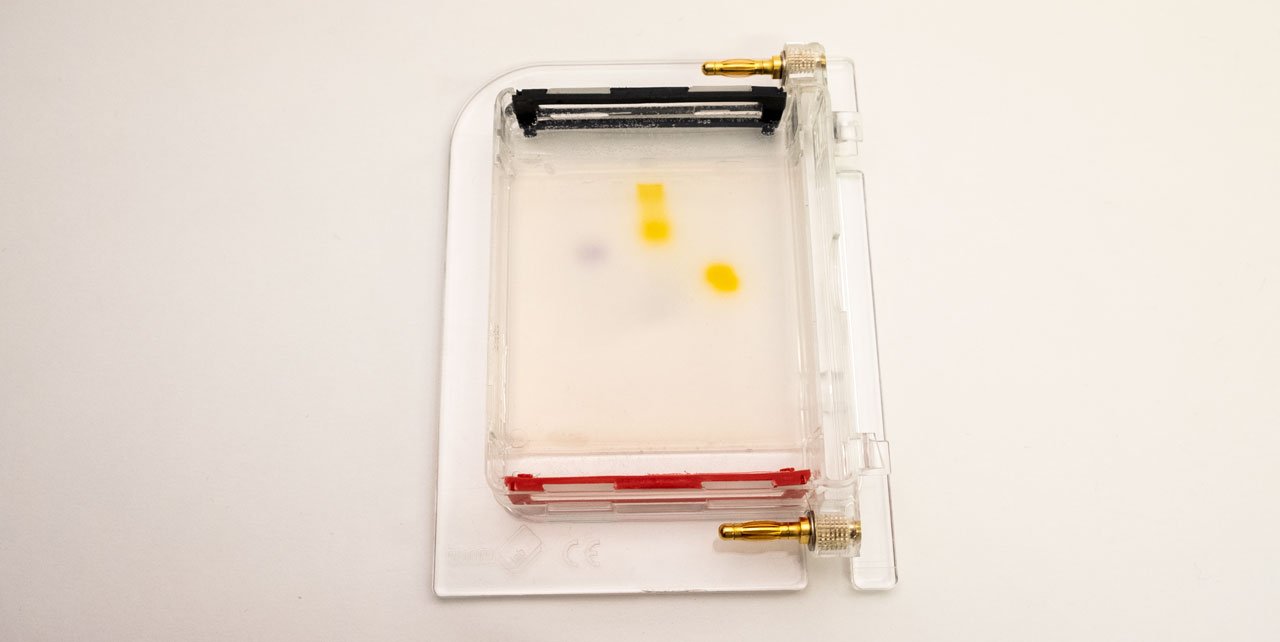
The gel (1) is a jelly-like substance made from agarose, a sugar polymer extracted from seaweed. The gel is immersed in a buffer solution and has electrodes (2 / 3) on either side, creating an electrical field. The gel is cast with small pockets close to the negative electrode. These are called wells (4). The samples, containing DNA pieces of different base pair sizes, are pipetted into the wells.
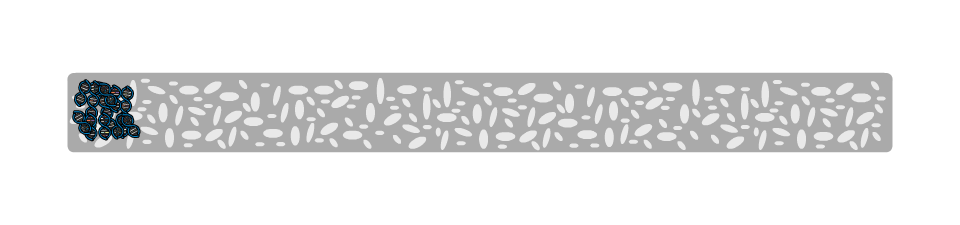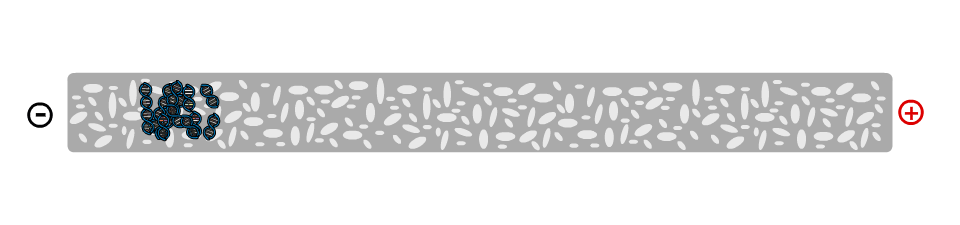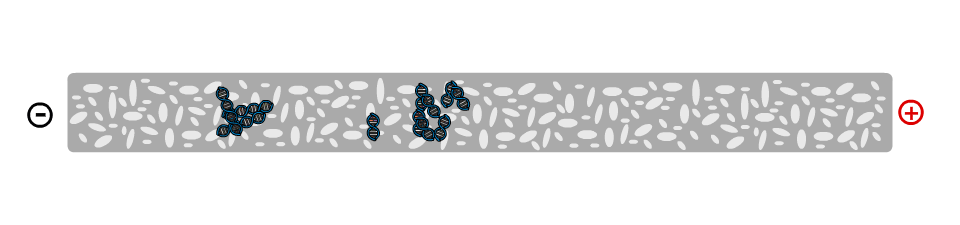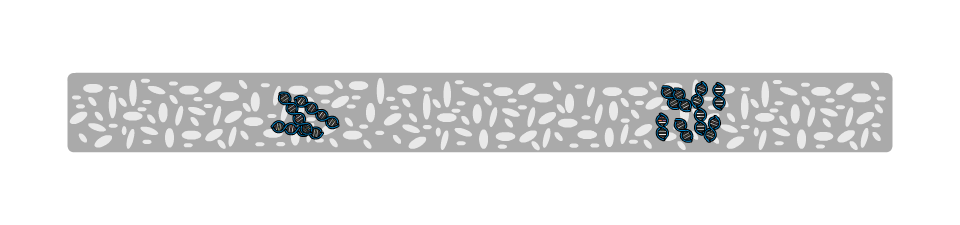
On a molecular level, the gel is not solid, but contains many small pores. Because the DNA molecule has a negative charge, due to its chemical structure, when a voltage is applied, the DNA fragments are pulled towards the positive electrode. The speed at which the DNA fragments travel through the gel depends on their size: Small pieces travel quickly, large pieces travel slowly. After some time, the DNA fragments have been separated and their size can be analysed. Depending on the samples used, the fragment size can give information about their genetic information.
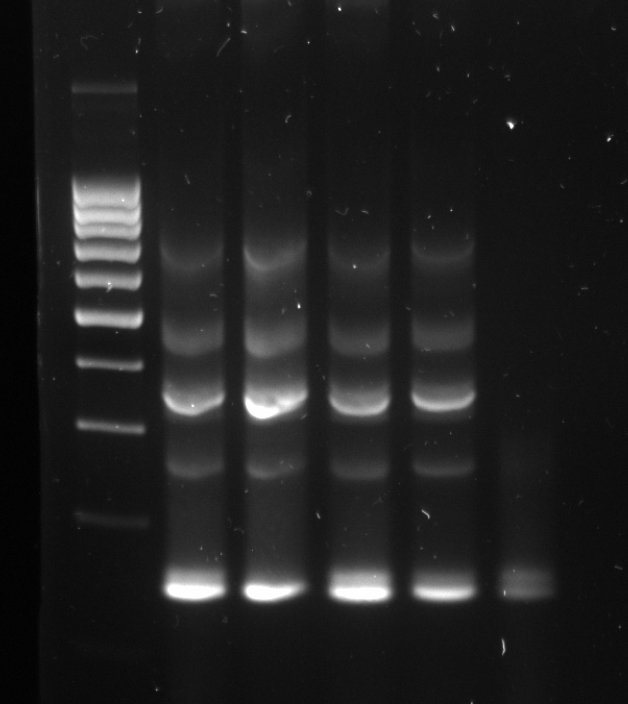
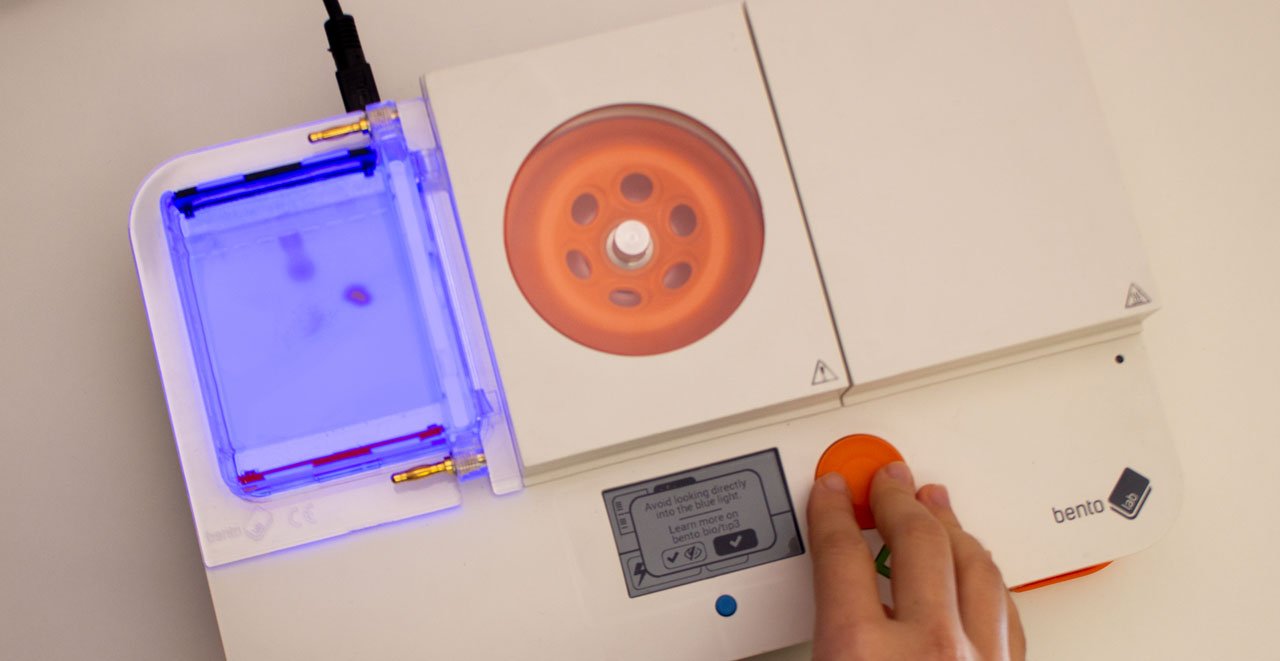
The gel can be infused with a DNA stain, which will bind to the samples. Using the transilluminator, the samples can then be made to fluoresce, so that they become visible.A typical gel picture looks like the picture above. From each well, the DNA fragments have travelled in their lane have been separated by their size. In the left lane, a DNA ladder has been used. This is a synthetic DNA mixture with fragments of known sizes, which is used as a ruler for the samples.