The least invasive sources of DNA are small feathers plucked from a bird’s breast. This extraction protocol will not work for moulted feathers.
Use a new scalpel/razor blade on a clean surface (e.g. a clean piece of paper towel on top of cutting mat/non-scratch surface) to prepare a very small section of feather approximately 2 mm3 diameter. For very small feathers include the base part of the feathers. For larger feathers cut a section very near the junction of the base and the first feather barbs – this area contains a DNA-rich blood clot (Horváth et al. 2005).
Place the sample in a PCR tube labelled on the top and side in permanent marker. To avoid sample cross-contamination, wash hands or change gloves between feathers and use a new scalpel/razor blade and piece of paper towel each time.
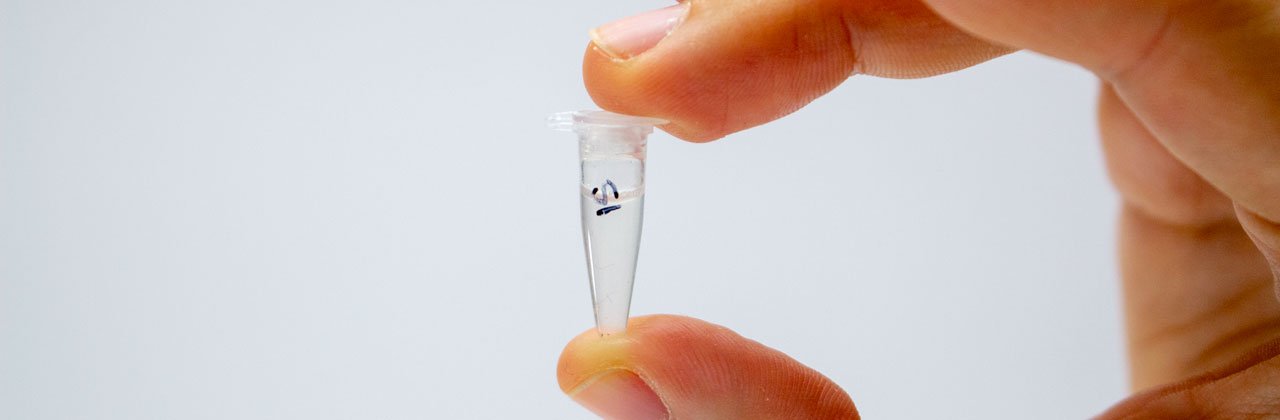
Even if you only have one sample, it’s good practice to label the tube clearly with a unique identifier for the feather used. It’s also a good idea to mark on the tube the date and to keep a note somewhere of which samples were prepared, and when.